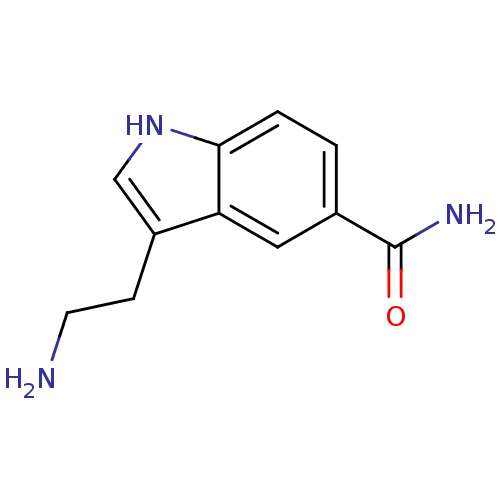
BDBM21392 3-(2-aminoethyl)-1H-indole-5-carboxamide::5-CT::5-CT,DP::5-carboxamidotryptamine::CHEMBL1256863::CHEMBL18840::[3H]-5-CT::[3H]-5-Carboxamide tryptamine::[3H]-5-Carboxyamidotryptamine::[3H]-5-carboxamidotryptamine
SMILES NCCc1c[nH]c2ccc(cc12)C(N)=O
InChI Key InChIKey=WKZLNEWVIAGNAW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 21392
Found 11 hits for monomerid = 21392
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
Mayo Foundation
Curated by PDSP Ki Database
Mayo Foundation
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
Mayo Foundation
Curated by PDSP Ki Database
Mayo Foundation
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
Mayo Foundation
Curated by PDSP Ki Database
Mayo Foundation
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
Mayo Foundation
Curated by PDSP Ki Database
Mayo Foundation
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
Mayo Foundation
Curated by PDSP Ki Database
Mayo Foundation
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
Mayo Foundation
Curated by PDSP Ki Database
Mayo Foundation
Curated by PDSP Ki Database
Affinity DataIC50: 3.40nMAssay Description:In Vitro Binding affinity againist 5-hydroxytryptamine 1D receptor by displacing [125I]GTI from pig caudateMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1D(Sus scrofa)
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.98nMAssay Description:Binding activity against 5-hydroxytryptamine 1D receptor from pig caudate membrane using [3H]5-HT as radioligandMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
Mayo Foundation
Curated by PDSP Ki Database
Mayo Foundation
Curated by PDSP Ki Database
Affinity DataKd: 1.60nMAssay Description:Binding affinity for 5-hydroxytryptamine 1D receptor was determinedMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1D(Sus scrofa)
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Displacement of radioligand [3H]5-HT binding to 5-hydroxytryptamine 1D receptor in pig caudate membraneMore data for this Ligand-Target Pair